If one mole of carbon contains “x” atoms, what is the number of atoms contained in 12 g of
magnesium (Mg)?
| A. x | B. 0.5x |
| C. 2x | D. 1.5x |
B
Which term is same for one mole of oxygen and one mole of water?
| A. volume | B. mass |
| C. atoms | D. molecules |
D
The electronic configuration of an element is 1s2 2s2. An atom of this element will form an
ion that will have charge
| A. +1 | B. +2 |
| C. +3 | D. -1 |
B
What is the mass of carbon present in 44 g carbondioxide (CO2)?
| A. 12 g | B. 6 g |
| C. 24 g | D. 44 g |
A
What is the mass of 4 moles of hydrogen gas?
| A. 8.064 g | B. 4.032 g |
| C. 1 g | D. 1.008 g |
A
How many moles of molecules are there in 16 g oxygen?
| A. 1 mole | B. 0.5 moles |
| C. 0.1 moles | D. 0.05 moles |
B
A compound with chemical Na2CX3 has formula mass 106 amu. Atomic mass of element “X” would be
| A. 106 amu | B. 23 amu |
| C. 12 amu | D. 16 amu |
D
What is the formula mass of copper sulphate pentahydrate (CuSO4.5H2O)?
[Atomic masses of copper (Cu) = 63.5 amu, sulphur (S) = 32 amu, oxygen (O) = 16 amu, hydrogen (H) = 1amu]
| A. 159.5 amu | B. 185.5 amu |
| C. 249.5 amu | D. 149.5 amu |
C
The diagrams below represent particles in four substances. Which box represents the particles of nitrogen?
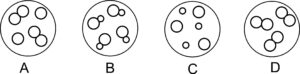
C
Which of the following lists contains only elements?
| A. air, water, oxygen | B. hydrogen, oxygen, brass |
| C. air, water, fire, earth | D. calcium, sulphur, carbon |
C
